


seek and you will find
- …


seek and you will find
- …

SeekInCureTM
Pan-cancer, non-invasive recurrence monitoring

Cancer recurrence is a leading cause of death among cancer patients. Even those who have undergone radical surgery in the early stages are at a certain risk of recurrence. Therefore, early detection of cancer recurrence signals and timely therapeutic intervention can help reduce the risk of death.
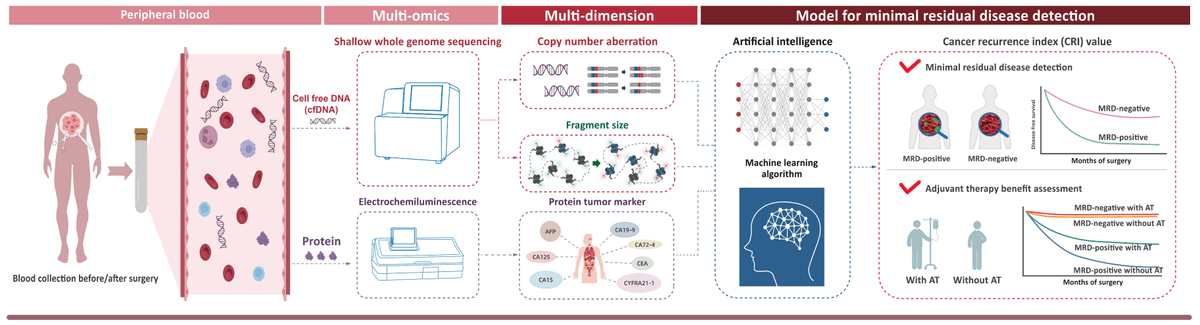
SeekInCureTM is a molecular residual disease (MRD) assay applied for monitoring cancer recurrence and evaluating risk in postoperative patients.
Pan-cancer
Non-invasive
MRD detection
Recurrence pridiction
Highlights
- SeeklnCureTM takes a panoramic view of cancer signatures in blood and a multi-omics approach incorporating genomic and epigenetic alterations in conjunction with clinically validated protein biomarkers.
- Equipped with proprietary artificial intelligence (AI)- and big data-driven cancer recurrence index (CRI) algorithm, it generates a CRI index to reflect cancer siginals.
- SeeklnCureTM is meant to be used serially to detect recurrence earlier.
Testing time

After Surgery,
check if there are cancer signs remaining, evaluate the need for adjuvant chemotherapy.
Assessing therapy efficacy,
evaluate if the adjuvant chemotherapy is effective, and determine when to escalate or adjust the treatment accordingly.
Early recurrence detection,
surveillance with greater sensitivity to detect signs of recurrence earlier than current standard of care tools.
Specifications
◉ Indicated subjects: cancer patients who undergone curative surgery
◉ Sample requirement: 8 mL peripheral blood
◉ Result readout: cancer recurrence index (CRI)
◉ Turnaround time (TAT): 10 working days

How it works

Order
Consult your doctor to order through a local healthcare partner

Blood draw
8 mL of peripheral blood is collected and delivered to the central lab

Testing
Detection is performed by combining immunoassay and next-generation sequencing (NGS) platforms

Reporting
Results are ready within 10 working days after the sample arrives at the central lab
Contact Us
10320 Camino Santa Fe, Suite G
San Diego, CA 92121
United States
info@seekincancer.com
© 2024 SeekIn Inc.
All Rights Reserved.



