


seek and you will find
- …


seek and you will find
- …

LungCanSeekTM
Blood-based lung cancer early detection service
Lung cancer is the leading cause of cancer mortality worldwide. The 5-year survival rate for advanced lung cancer is less than 20%, while the survival rate for early-stage lung cancer is over 60%. Early screening for lung cancer is key to extending patient survival and lowering mortality rates.
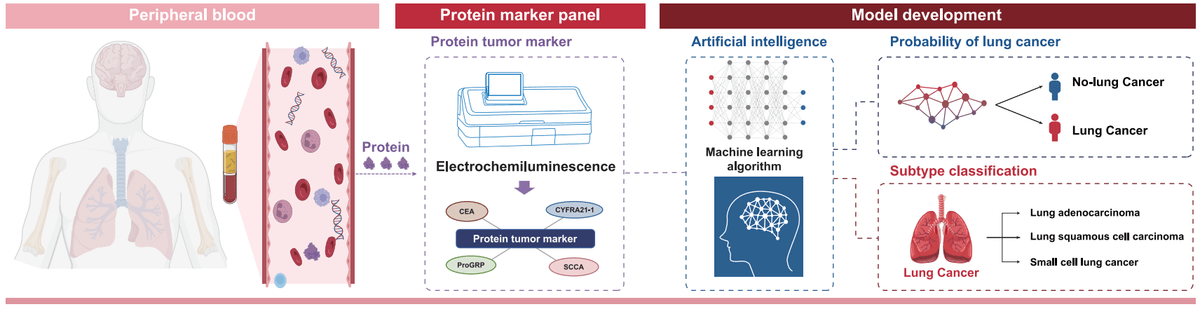
LungCanSeekTM is an artificial intelligence (AI)-driven early lung cancer screening service designed to identify cancer at its curable stage, enabling timely intervention to improve clinical outcomes.
Al-powered
Non-invasive
Early detection
Cost-effective
Highlights
- LungCanSeekTM calculates a probability of lung cancer (POLC) index by precisely analyzing the levels of four lung cancer related protein tumor markers (PTMs).
- When cancer signal is detected, LungCanSeekTM can predict main lung cancer subtypes: lung adenocarcinoma (LUAD), lung squamous cell carcinoma (LUSC), and small cell lung cancer (SCLC).
Two-step approach for lung cancer early detection
Use LungCanSeekTM first, followed by low-dose computed tomography (LDCT) for individuals who test positive - offering a more accurate and cost-effective alternative to LDCT screening alone.
- >10-fold reduction in false positives
- >2.5-fold reduction in screening costs
Indicated subjects

Smoked at least a pack a day for 20 years
Significant secondhand smoke exposure
High exposure
to indoor pollutants
(e.g., cooking
oil fumes)
Exposure to environmental carcinogens
(e.g., radon, asbestos)
History of chronic obstructive pulmonary disease (COPD) or pulmonary fibrosis
Family history of lung cancer
Specifications
◉ Sample requirement: 4 mL peripheral blood
◉ Result readout: probability of lung cancer (POLC)
◉ Turnaround time (TAT): 5 working days
How it works

Order
Consult your doctor to order through a local healthcare partner

Blood draw
4 mL of peripheral blood is collected and delivered to the central lab

Testing
Detection is performed using immunoassay platforms, such as Roche Cobas

Reporting
Results are ready within 5 working days after the sample arrives at the central lab
Please review the instructions to gain a better understanding of LungCanSeekTM and assess whether it meets your needs.
Contact Us
10320 Camino Santa Fe, Suite G
San Diego, CA 92121
United States
info@
© 2024 SeekIn Inc.
All Rights Reserved.

