seek and you will find
- …


seek and you will find
- …

SeekInClarity®
Pan-cancer, pan-indication treatment response monitoring

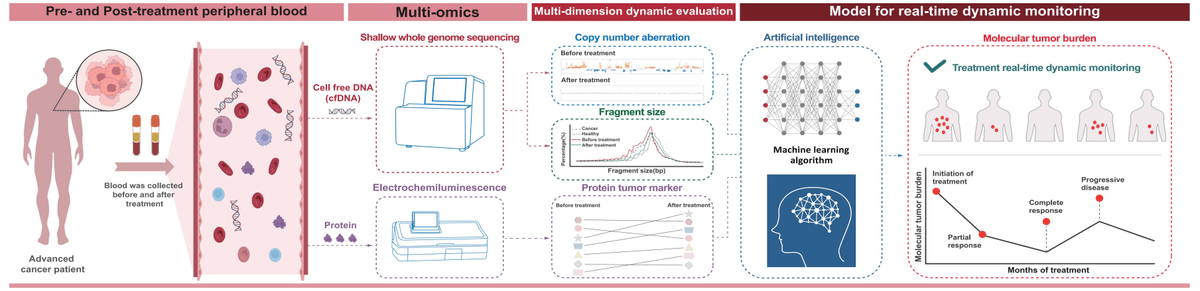
Therapeutic efficacy monitoring is essential in cancer care, assessing tumor burden changes, treatment response, early drug resistance, and long-term efficacy. This enables personalized precision medicine to enhance outcomes and patient quality of life.
SeekInClarity® is a non-invasive, pan-cancer, pan-indication solution for advanced cancer patients. By detecting molecular signals earlier than traditional methods, it supports timely clinical decisions to improve patient outcomes.
Pan-cancer
Pan-indication
Early assessment
Progression prediction
Highlights
- Circulating tumor DNA (ctDNA) and proteins were analyzed from a single blood draw using a multiomics approach.
- Equipped with proprietary artificial intelligence (AI) and big data-driven molecular tumor burden (MTB) algorithms, it generates MTB scores that reflect cancer signals.

Testing time

Before treatment,
use to set a personalized baseline level.
After treatment,
compare with the baseline to check if the treatment is working well.
Early progression detection,
surveillance with greater sensitivity to detect cancer progression earlier than current standard of care tools.
Specifications
◉ Indicated subjects: patients who are diagnosed with advanced cancer
◉ Sample requirement: 8 mL peripheral blood
◉ Result readout: molecular tumor burden (MTB)
◉ Turnaround time (TAT): 10 working days

By showing the increase or decrease in MTB between two tests, it reflects changes in cancer signals and highlights the effectiveness of treatment.
How it works

Order
Consult your doctor to order through a local healthcare partner

Blood draw
8 mL of peripheral blood is collected and delivered to the central lab

Testing
Detection is performed by combining immunoassay and next-generation sequencing (NGS) platforms

Reporting
Results are ready within 10 working days after the sample arrives at the central lab
Please review the instructions to gain a better understanding of SeekInClarity® and assess whether it meets your needs.
Contact Us
10320 Camino Santa Fe, Suite G
San Diego, CA 92121
United States
info@
© 2024 SeekIn Inc.
All Rights Reserved.