


seek and you will find
- …


seek and you will find
- …

LeukoPrint®
Hematologic Malignancy Molecular Karyotyping Test
LeukoPrint® is a molecular karyotyping test designed to complement conventional cytogenetic approaches in hematologic malignancy diagnostics. lt employs shallow whole-genome sequencing (sWGS) to deliver comprehensive, high-resolution genome coverage for the detection of clinically significant copy number aberration (CNA) and copy neutral loss of heterozygosity (CN-LOH), providing advantages over traditional karyotyping and fluorescence in situ hybridization (FlSH).
Molecular karyotyping
Precision diagnosis
Risk stratification
Therapy guidance
Highlights
- LeukoPrint® performs shallow whole-genome sequencing (sWGS, at 1× coverage) on bone marrow samples to identify genome-wide CNA and CN-LOH.
- When combined with traditional cytogenetic methods, it enables more accurate diagnosis,classification, risk-stratification, and ultimately guides risk-adapted therapy for hematologic malignancies.
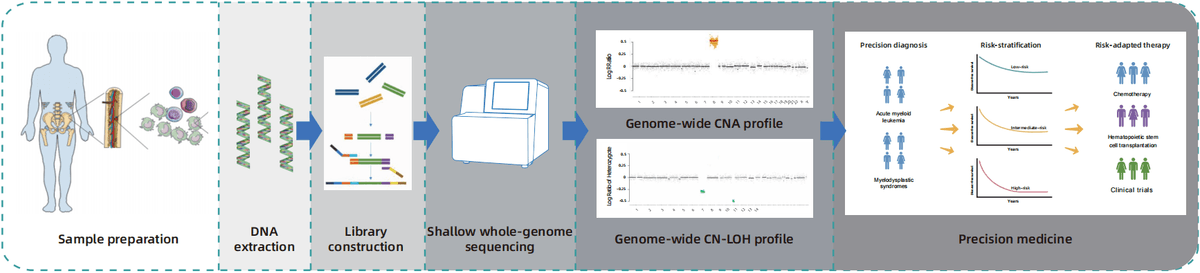
Advantages
◉ No cell culture
Faster results, no culture failures.
◉ Genome-wide CNA/CN-LOH
Sensitive, automated detection of key abnormalities missed
by standard methods.
◉ Greater diagnostic yield
Accurately detects small CNAs (≥1 Mb) even in low-tumor or
ambiguous samples.
◉ Clinical actionability
Supports risk-adapted therapy with ELN/IPSS-/mSMART
integration.
Testing time
First-line diagnostic adjunct
enhances sensitivity in
suspected hematologic
malignancies.
Karyotyping failure rescue
provides full genomic profiling when standard
karyotyping fails.
Complex case resolution
reveals cryptic abnormalities when karyotyping/FISH
are inconclusive.
Critical variant detection
identifies CN-LOH
undetectable by
karyotyping/FISH.
Specifications
◉ Indicated subjects:Patients with suspected or confirmed hematologic malignancies
◉ Sample requirement:2-4 mL bone marrow
◉ Result readout:CNA and CN-LOH
◉ Turnaround time (TAT):10 working days
How it works

Order
Consult your doctor to order through a local healthcare partner

Blood draw
2-4 mL bone marrow is collected and delivered to the central lab

Testing
Detection is performed using next generation sequencing (NGS) platform

Reporting
Results are ready within 10 working days after the sample arrives at the central lab
Please review the instructions to gain a better understanding of LeukoPrint® and assess whether it meets your needs.
References
Contact Us
10320 Camino Santa Fe, Suite G
San Diego, CA 92121
United States
info@seekincancer.com
© 2024 SeekIn Inc.
All Rights Reserved.

